Blog
Membrane Electrode Assembly in Fuel Cell
As the core component of proton exchange membrane fuel cells (PEMFCs), membrane electrodes assembly(MEA) provide a place for reactions in three-phase material boundaries, ensure a complex heat and mass transfer process. The electrical conductivity, service life, and production cost of mold electrode assembly directly affect the commercialization level of fuel cells. A single cell generally consists of a membrane electrode, a gas diffusion layer(GDL) and a cathode/anode bipolar plate. It’s an energy conversion device that converts chemical energy from hydrogen fuel and oxidants into electrical energy. In practical application, multiple single cells are designed to form a fuel cell stack to meet various power supply requirements, which has many advantages in environmental protection, high energy density and smooth operation, and has become a hotspot technology researched by governments and companies around the world.
Composition of membrane electrode for PEMFC
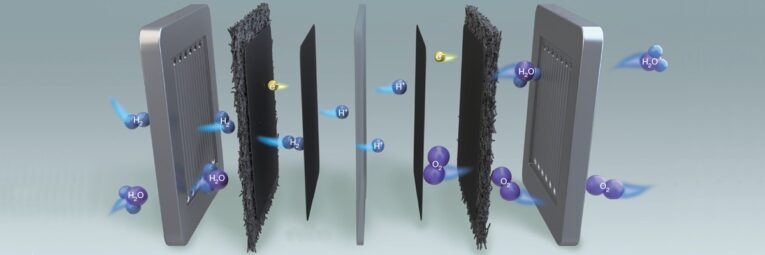
As shown in the following figure, MEA mainly consists of cathode/anode catalytic layer(CL), proton exchange membrane(PEM) and cathode/anode gas diffusion layer(GDL), and GDL includes carbon cloth, carbon paper and micro-porous layer(MPL) coated on the surface. PEM provides a channel for proton transfer from the anode to cathode, and reduce mass transfer resistance. Cathode and anode catalytic layer stick to both sides of PEM respectively. The main component includes PT/C catalyst mixed with perfluorosulfonic acid ionomers, provide sufficient areas for three-phase material transport(Pt nanoparticles, ionomers, gas contact) and electrochemical reactions. Due to different technology, gas diffusion layer generally is made from carbon paper, carbon cloth, carbon felt, and other materials. In addition, metal mesh, metal fiber can also be used for raw materials. GDL not only play a role in electron conduction, reaction gas diffusion and drainage, but also contact with the external bipolar plate, undertake function of mechanical support. The interface structure and functional coordination between layers have a significant impact on final performance of MEAs.
Working principle of MEA
During the operation of proton exchange membrane fuel cell, H2 enters through the flow path of anode bipolar plate and reaches the catalytic layer through anode diffusion layer and microporous layer. Under the action of a catalyst, an hydrogen oxidation reaction(HOR) occurs at anode, h2 is broken into protons H+ and release electrons, which pass through the PEM arrive at the surface of catalytic layer at cathode. At the same time, oxygen gas from cathode which pass through gas diffusion layer is also adsorbed on catalytic layer at cathode, under the action of the cathode catalyst, oxygen reduction reaction(ORR) occurs between oxygen and H+ from cathode, and produce water. The electrons form a directional current at external circuit to supply electrical energy for load, and excess water and reaction gas are discharged through the electrodes together. The entire operation process actually undergoes a three-phase transport: materials, protons and electrons. Materials mainly include gaseous products and water transported in CL and GDL. Protons are conducted along the ionomer in CL, pass through PEM from anode to cathode, and electrons are transported in CL based on the electrical conductivity of PT/C catalyst, passing through GDL to the external circuit. The results indicate that the performance of PEMFC is directly determined by electron/proton conductivity, gas diffusion ability and hydrophobicity of membrane electrode assembly.
Preparation methods for membrane electrode assembly
At presant, the manufactories which master leading-edge membrane electrode technology are mainly distributed in the United States and Japan. However, most of MEA products are not on sale due to market strategy, and the scarce product supply leads to high market prices. Therefore, the domestic demand for membrane electrode products have become increasingly urgent. Based on different CL supports, common MEA preparation technology methods include GDE type and CCM type. More content are detailed in articles “CCM-MEA in Proton Exchange Membrane Fuel Cell” and “GDE-MEA in Proton Exchange Membrane Fuel Cell“, and no more tautology here. According to different coating methods of catalyst active components, the following are three main production methods: transfer printing method, electrochemical deposition method and ultrasonic spraying method:
1) Transfer printing method.The advantage is that proton exchange membranes don’t need to contact solvents, avoid swelling and wrinkling issues due to water absorption, effectively improve the performance of CCM membrane electrode. The disadvantages include uneven distribution of the catalyst active components, low utility of catalytic, peeling problems during hot pressing process, etc.
2) Electrode deposition method. Generally occurs in electroplating baths, electric field can be used to uniformly deposit the catalyst on the core area reaction of MEA. It can also electrolyze Pt from solution to produce more contact with Nafion membrane, reduce loading amount of Pt while no need to degrade the performance of fuel cell. The disadvantage is that the catalyst particles are uneven in size and easy to form agglomeration.
3) Ultrasonic spray method. First the catalyst materials are dispersed via ultrasonic cavitation effect, and then ultrasonic sprayed onto surface of GDL or PEM. The ultrasound method can spray tiny and uniform particles, and effectively avoid agglomerating issues, greatly save the cost of catalyst. Sonicating method is easy for operation and with high level of automation, suitable for mass production for membrane electrode. The disadvantage is that the energy consumption is relatively high.
Besides, there are many other preparation methods such as scratch coating, screen printing, sputtering, etc. Due to the limitation of length, don’t explore it. With the rapid growth of fuel cell vehicles and valued by more and more governments and factories around the world, high performance, low cost, high durability, low platinum (Pt) loading, have become the important research direction on MEA. The application of membrane electrode assembly in proton exchange membrane fuel cells will gradually realized industrialization.
About Mr. Zhou
Search
Recent Posts
-
Manufacturing Process of Ca... 11/28/2024
-
Application of Flexible Gra... 05/14/2024
-
PEM Water Electrolysis for ... 04/12/2024
-
Application of Bipolar Memb... 01/09/2024
-
Membrane Electrode Assembly... 11/27/2023
Categories
- All Posts (24)
- Flow Battery (11)
- Battery Material (20)
- Bipolar Plate (13)
- Membrane (3)
- Felt Electrode (1)
- MEA (3)
- Fuel Cell (5)
Contact Info.
Recent Post
-
Manufacturing Process of Ca... 11/28/2024
-
Application of Flexible Gra... 05/14/2024
-
PEM Water Electrolysis for ... 04/12/2024
-
Application of Bipolar Memb... 01/09/2024
-
Membrane Electrode Assembly... 11/27/2023